Features
Life changing technical innovation in type 1 diabetes treatment uses artificial intelligence to determine and administer insulin automatically
04 June 2019
Erik Huneker, co-founder of European SME Diabeloop, speaks to 4iP Council about his company's innovation.

Erik Huneker, Diabeloop co-founder
Founded in 2015, Diabeloop develops disruptive technological innovations for type 1 diabetes treatment. Here we talk to Erik Huneker, Diabeloop co-founder, about the company’s groundbreaking DBLG1™ device, which uses artificial intelligence to determine and administer insulin automatically. This product, given the CE mark in November 2018, was launched in the spring and will be made available on prescription in Europe as it becomes integrated within national healthcare reimbursement systems.
How does the DBLG1 System work and what are the main advantages of its approach over traditional methods?
The DGLB1 System is an external medical device that connects 3 components: a continuous glucose monitor (CGM), a patch insulin pump and a locked-down handset hosting the algorithm developed by Diabeloop and user interface.
Every 5 minutes, a glucose measurement is transmitted via Bluetooth technology to the handset. DBLG1 artificial intelligence analyses data in real time and takes into account the patient’s physiology, history and data entries (meals or exercise) to determine the correct dose of insulin to administer.
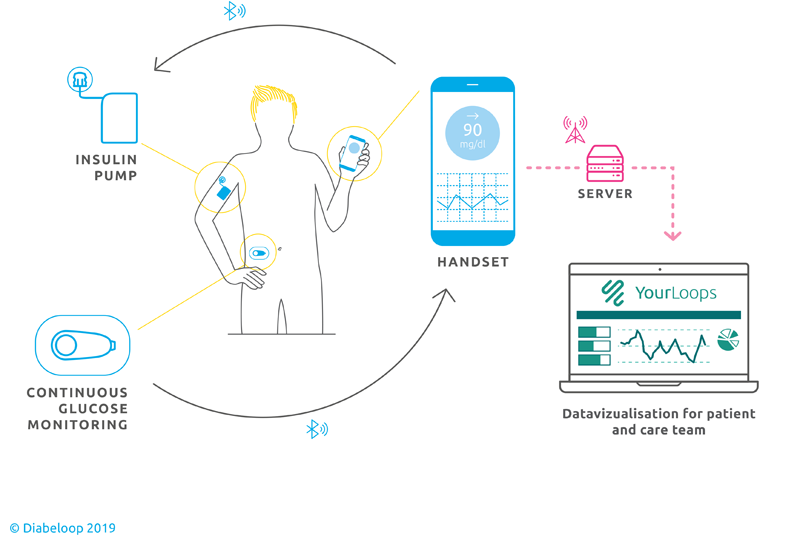
DBLG1 promises patents fewer daily hypoglycaemia attacks, lowers the risk of complications related to diabetes and reduces the hassle of self-monitoring and self-administration. It works together with the Dexcom G6 sensor and Kaleido pump manufactured by Vicentra.
What is the core innovation in DGLB1 and how is it patented?
The core innovation in DGLB1 is the algorithm that analyses all the data gathered by the system and decides what to do with the insulin level. The system isn’t completely intervention free, as the patient still needs to validate when he or she is eating (a functionality requested by patients in clinical trials as an additional safeguard) and change the infusion set, but it is able to adapt to the patient’s physiology, lifestyle and preferences and learn from the past, taking away a lot of the thinking around dosage and timing that that is very burdensome psychologically to type 1 patients. Some very complex factors need to be accounted for to ensure safe personalisation and DBLG1 delivers this.
Algorithms cannot be patented but you can patent the mechanisms behind them. There are multiple algorithms working at different layers such as safety layers, a layer that looks at the physiology of the patent using machine learning, what we call an expert system layer, that identifies unusual happenings. There is a lot of IP behind these mechanisms and how they interact.
We worked with the French research institute CEA and our own IP lawyers to develop Diabeloop’s patents some of which we own jointly with CEA and leverage through an exclusive worldwide license. Others are owned entirely by us.
Who invented the innovation/s behind the Diabeloop project, and how has the innovation evolved to its current the stage?
Dr. Guillaume Charpentier saw the need. He is a diabetologist who also runs CERITD, a non-profit with both patents and doctors focused on prevention and diabetes treatment improvement. Charpentier teamed Diabeloop up with CEA to begin the algorithm development and now leads the Diabeloop Board.
CERITD is funded through donations. It spends €1-2 million annually on type 1 and 2 research, supporting 50 researchers. Learn more here.
Where did Diabeloop’s funding come from?
Initial funding came from two sources, the non-profit sector and public funding mechanisms for life sciences. Afterwards, venture capitalists and the BPI France invested. Other banks too provided loans. We have also benefited from important European Union research funds. EIT Health awarded us a first grant in 2017 to develop a kit that is adapted to childrens’ user experiences -clinical trials begun this spring - and a second in 2019 to personalise further and address the needs of adolescents. A significant portion of adolescents go into denial mode on their diabetes, running into a treatment wall because they want to be seen as ‘normal’.
We very are proud of our work to make DBLG1 adaptable to different age groups and physiological needs. Too few medical devices have a special user experience for kids.
We also received European Union grants from the Eurostars Programme and the SME Instrument
See 4iP Council’s interview with Catherine Eginard, Head of Sector, H2020, for more detail on the criteria for accessing SME Instrument funding.
The development cost of DBLG1 is not high when compared with the amounts spent by some of our US counterparts where costs can rise to over $80 million.
Was IP an important asset for you from the beginning and how are you leveraging it now?
IP is absolutely critical if you want an investor. Put simply, if you have not done your work on IP you will not get an investment. Our focus today is however on getting out into the market as soon as possible so our current IP strategy is all about managing our freedom to operate and securing investors. We are not looking at ways to leverage the IP to make money per se, although that may well change in the future.
What challenges do you face today as a start-up in pharma/medical devices field?
One of the biggest challenges we face is that not many people truly understand what it means to live with type 1 and, as a consequence, people find it difficult to understand how disruptive DBLG1 is in terms of increased autonomy. Patients involved in the clinical trials have said that using DBLG1 means they are not afraid to walk alone, that they have peace of mind because they are no longer being woken in the middle of the night with hypoglycaemia symptoms when blood sugar level get too low. What DBLG1 achieves is not perfect but it is life changing.
What factors have contributed most to your success?
Listening has contributed greatly to our success. We have really tried to understand the different physiologies of people with type 1. Each of our developers sat with patients to understand their need and 20 per cent of all of the people working for us are living with type 1 either directly or through family members. Our team is very strong too. We have the luxury of being able to offer work on a very interesting topic and, as a result, have been able to attract people who are really good not just in R&D but across all domains. And we’ve given the team the freedom to really contribute.
How would you like to see support for inventors in Europe evolve?
IP is a broken system in many ways and this is frustrating, but you have to live with it and learn how to work with it. Large companies have the ability to put out many patents that can be deliberately vague. These patents become a barrier to innovation as opposed to a form of protection. The system needs to monitor quality and precision.
There are challenges too in the nature of reimbursement systems: slow processes; a lack of uniformity across Europe. You can’t change these systems, but they do impact speed to market so you have to understand and work with them.
What insights has Diabeloop given you about turning an idea into a product that might help other inventors?
Get protection, find a good lawyer and make sure you know what needs to be done regarding the remit of protection. You can very easily run up a huge bill trying to enable freedom to operate. Remember too that you can’t change or avoid the system. It is necessary to spend the time it requires because you need to do it. Don’t be discouraged, IP is a strategy that needs to be played.
For further information on Diabeloop’s work and the launch of the DBLG1 System, visit www.diabeloop.com.
Author: Emma Bluck
The views expressed in this feature are those of the interviewee and may not reflect the views of 4iP Council or its members. The purpose of this feature area is to reflect thinking on the topic of intellectual property and enable open discussion.



